by Dave Arnold.
At this year’s Tales of the Cocktail, Eben Klemm, beverage director for BR Guests restaurants and the author of The Cocktail Primer: All You Need to Know to Make the Perfect Drink; Thomas Waugh, bartender extraordinaire at Death & Co; and I did a seminar called The Science of Stirring –a follow-up to last year’s presentation, The Science of Shaking. Rather than post a summary of the seminar, I’ve taken on the more ambitious task of summarizing everything I’ve learned about cocktail science over the past year.

I’m breaking this post into segments. The one below is about shaking, stirring, temperature and dilution; the next one addresses how you perceive temperature and dilution, texture, and notes on batching drinks. Stick with it, and at the end of the second segment you’ll get a bonus: Thomas Waugh talking about how all this science stuff affects a real live bartender.
For those of you without patience: the Short Story
Cocktail shaking is a violent activity. If you shake for around 12-15 seconds (though shaking longer won’t hurt), and if you aren’t too lethargic, neither the type of ice you use nor your shaking style will appreciably affect the temperature or dilution of your drink. Shaking completely chills, dilutes and aerates a drink in around 15 seconds, after which the drink stops changing radically and reaches relative equilibrium. Shaking is basically insensitive to bartender-induced variables. See my post on the Science of Shaking.
Stirring is different. Think of stirring as inefficient shaking. It can take over 2 minutes of constant stirring to do what shaking can accomplish in 15 seconds. No one stirs a drink for 2 minutes, so the drink never reaches an equilibrium point. All the bartender-induced variables – size of ice, speed of stirring, duration of stirring, etc. — make a difference in stirred cocktails, so bartender skill is very important in a stirred cocktail.
Because stirring doesn’t reach equilibrium, stirred drinks are warmer and less diluted than shaken cocktails. Stirred drinks, unlike shaken ones, are not aerated. Stirring does not alter the texture of a drink –it merely chills and dilutes. A properly diluted cocktail stored at -5 degrees Celsius in a freezer is indistinguishable from a properly stirred one.
Don’t believe me? The proof’s in the long story.
Long Story
The Equipment I Used for my Experiments.
I took temperature readings with a thermocouple. I drilled holes into the bottom of metal shakers, pint glasses, and Japanese crystal stirring vessels and inserted thin stainless steel thermocouples with ½ second response times. I sealed the bottom of the containers with Mighty Putty, which made them water-tight and allowed them to sit flat despite the thermocouples. God bless Mighty Putty, may Billy Mays rest in peace. I read the thermocouples using a Measurement Computing 8 channel thermocouple input module (Model USB-TC, a pretty good deal at $329). I recorded weights on a digital scale accurate to 0.1 gram.

A Preliminary Rant on the Temperature of Ice:
Fact 1: Ice at 0°C can chill an alcoholic drink well below 0°C. This fact is counter-intuitive to many, but is an irrefutable consequence of the laws of thermodynamics. The universe likes increased entropy. If you want an actual explanation, see my first post on Cocktail Science. For visual proof, I submit the following experiment:

I took ice from my freezer, put it in cold water, and allowed it to sit for 15 minutes. I then took some of the ice and water and put them into a mixing glass with a thermocouple and vigorously stirred for 120 seconds to ensure that everything was at 0°C. I drained the water from the ice, put the ice into a mixing glass with room-temperature vodka, and started stirring. Less than 30 seconds later my vodka was colder than 0°C.
Fact 2: Bar ice is almost always at 0°C unless it comes straight from the freezer. People have a hard time accepting this fact. As a test, I froze a large ice cube with a super-thin hypodermic thermocouple probe in the center. I put that ice cube, along with some run-of-the-mill ice cubes for insulation, into a blast freezer for 4 hours until everything was at -20 C. I then put the entire batch into a plastic container and waited. In under 20 minutes, the large ice cube was within 0.5 degrees of zero.

Why?: 1. The ice warms up so quickly because it is a very good conductor of heat – four times better than stationary water.  Unless water is moving (convecting), it isn’t a good conductor. 2. Ice has a low specific heat — i.e., it doesn’t take a lot of energy to heat it up. It takes twice the energy to heat a pound of water 1 degree than it does to heat a pound of ice. For more on specific heat, see the Anomalies of Water Page.
Fact 3: Even if your ice is below 0°C, it won’t chill a drink that much better than ice at 0°C. Ice’s tremendous chilling power doesn’t come from the energy required to heat it up, but from the energy required to melt it. It takes 0.5 calories to heat a gram of ice from -1°C to 0°C (this value is called the specific heat of ice,) but almost 80 calories to melt that same gram (this value is called the heat of fusion of water). To put it another way, melting 1 gram of ice provides the same chilling power as bringing that same gram of ice from -160°C to 0°C. If you chill a cocktail with 150 grams of ice at -10°C, the amount of extra chilling power from the super-frozen ice is equivalent to melting only 9.5 grams of ice.
An experiment repeatedly conducted by Eben Klemm and Thomas Waugh indicates that super-frozen ice may actually chill drinks slower than ice at 0°C (even though the drinks reach a slightly lower final temperature). I repeated the experiment once with them and once by myself, and my results matched theirs (see the chart below). I am not certain why, but my guess is that melting ice chills three ways: through conduction, convection of the drink, and convection of the melt-water; whereas chilling without melting only uses conduction and convection of the drink.

The Fundamental Law of Cocktails:
Assumption: Bar ice is at 0°C.
Law: There is no chilling without dilution. There is no dilution without chilling. The only way ice can melt is by absorbing energy from its surroundings –by chilling. Chilling and dilution are two sides of the same coin. This observation seems trivial, but the consequences are deep. For instance, many bartenders like to serve drinks with big rocks of ice because the big ice will dilute the drinks less over time. This is true, but it also will not keep the drinks as cold. You can’t have it both ways: you can’t keep a drink as cold as possible while also diluting it as little as possible. Personally, if I were served an old fashioned I’d rather have the big rock and let the drink get a little warmer (it can get above 0°C pretty quickly when served with big ice) than let it get too watery.
Later in this post you’ll see some striking proofs of the fundamental law.
As a side note, not all chilling makes your drink colder. Some chilling power is consumed in chilling your shaking or mixing vessel. This energy isn’t negligible –in stirred drinks especially, the type of container you use makes a difference. Metal shakers heat up and cool down quickly using minimal energy –they don’t affect your drink much. Pint mixing glasses have more thermal mass than a shaker and absorb some energy from your drink. Heavy Japanese crystal mixing glasses absorb the most of all. Pre-chilling those glasses before making your drink mitigates these effects and makes them as good as –or better than, an un-chilled metal shaker. Some chilling power is also consumed overcoming the friction of mixing or shaking your drink, but this energy loss is negligible (for proof see the second experiment in The Science of Shaking II). Lastly, some energy is lost to the surrounding environment. I ignore this energy loss, because the amount of energy lost during the mixing and shaking process is small. On the other hand, it is this energy loss to the environment that turns a drink to dreck if it sits around waiting to be drunk.
An Apparent Exception to the Fundamental Law: The Surface Water Problem.
Everyone thinks that small ice cubes and crushed ice will inherently dilute a drink more than big ice cubes will. Here’s what’s really going on: crushed ice has a lot more water trapped on its surface than the big ice does. Big ice cubes have less surface area per gram than small cubes do. Bar ice at 0°C has water on its surface, so big ice cubes have much less surface water per gram than crushed bar ice does. This initial excess water dilutes your drink right away. After the initial dilution, the big ice and little ice go back to having the same chilling power. If you shake or spin the extra water off your small ice before you make a drink, it actually won’t dilute your drink any more than big ice will. For proof see my post: Does Crushed Ice Dilute More?
Chilling –Shaking vs. Stirring:
I have shown that ice can chill an alcoholic drink well below freezing. Just how far below freezing is dependent on a number of variables: the initial temperature of the drink, the initial alcohol content of the drink, and how efficient your chilling is. The amount of ice you use doesn’t really matter (so long as you use enough –see the Assumptions section of the Science of Shaking II post. Most drinks start at room temperature (unless you are making gin and tonics –shame on you if those ingredients are room temp). The initial alcohol content is determined by the recipe you use. The only variable you really get to control is the efficiency of your chilling.
When chilling, stirring is just inefficient shaking.
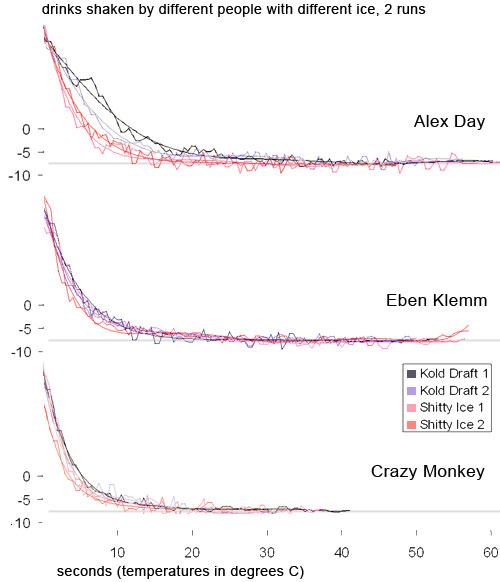
Shaking is so violent that it accomplishes everything it needs to in about 15 seconds. After 15 seconds, the drink won’t chill much more, and the drink won’t dilute much more. It’s reached relative equilibrium. The type of ice you use, how hard you shake (within reason –lazy shaking is no bueno), the style of shake, and how long you shake after 15 seconds doesn’t really matter. The long-winded proof of is in Science of Shaking II, but here is a chart from that post showing chilling curves for different bartenders and different types of ice:
Stirring is much more mellow than shaking. To stir a drink to the same temperature plateau that a shaken drink reaches in 15 seconds, you might need to stir 1-2 minutes. No one stirs this long, which means stirred drinks never reach equilibrium, which means stirring is complicated. Here, a comparison of stirring versus shaking:
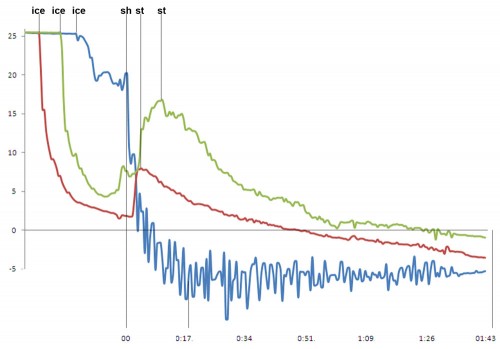
You can see large temperature drops in the stirred drinks when the ice is dropped (denoted by “ice†in the chart). This is because the ice is actually hitting the thermocouple. The temperature rises sharply when the drink is actually stirred (denoted by “st†in the chart). The beginning of shaking is denoted by “sh.†The temperature swings are large in the shaken drink because the drink and ice are sloshed on and off the thermocouple. Equal weights of uncracked Kold Draft ice cubes at 0°C  were used for all three drinks. The initial volume and temperature of the drinks was identical. Notice how fast the shaking chills. The drink hits 0°C in under 10 seconds and has plateaued at -7°C in less than 17 seconds. Fast stirring gets the drink below 0°C in about 45 seconds and hits -3°C in about 1 minute 45 seconds. The slow stirring takes almost a minute and 15 seconds to get to 0°C. How you stir makes a difference.
Here is a comparison of stirring using three different sizes of ice all at 0°C:
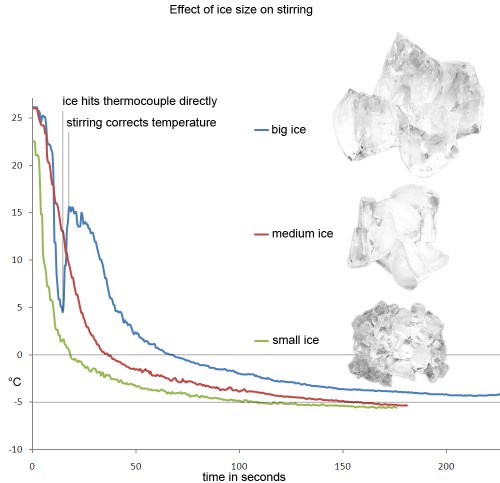
For scale, the “medium†sized cubes are the standard cubes produce by my home fridge’s ice cube maker. All of the pictures are to scale. The big ice is really bad at chilling quickly. The small ice gets the drink below 0°C in a snappy 20 seconds –the big ice takes well over a minute. Notice that the medium ice is only about 20 seconds behind the small ice in getting to 0°C, but 2 minutes behind the small ice in reaching a -5°C plateau. It is more difficult for larger ice to chill those last couple of degrees. The big ice plateaus in a whopping 220 seconds at about -4°C. Presumably, the length of time I had to stir and the extra energy from stirring so long is what prevented me from reaching -5°C like the other two drinks. To demonstrate that small ice chills more effectively, Ryan Fitzgerald, from Beretta in San Francisco, volunteered to stir a drink with very finely crushed ice during our seminar at Tales of the Cocktail. We spun the ice in a salad spinner to make sure it was “dry.†The tiny ice was so efficient that it chilled as fast as shaking. His drink made it to -5°C in under 10 seconds and went all the way to -7°C in under 15. Unfortunately, we all decided that his drink was too diluted. All that extra chilling came at the expense of too much dilution. It would have been fine for a shaken drink, but not for a stirred one.
In light of the above chart, the common bar practice of cracking large ice cubes with the back of a spoon for stirred cocktails makes a lot of sense. Un-cracked big ice is too inefficient at chilling; but smaller, more efficient ice might be carrying a lot of water on its surface. Cracking a big cube increases your surface area without increasing the amount of surface water.
The upshot? In stirring, the type of ice you use makes a big difference.
Two Visual Proofs of the Fundamental Law:
Proof 1: If you stir two drinks with different size ice cubes, but pour them out when they reach the same temperature, they will have the same dilution even though they were stirred for very different lengths of time. The drinks will be identical!

I pulled the drinks when they reached -0.6°C. Notice this temperature is a far cry from the -5°C plateau temperature I could have achieved by stirring for a long, long time, but stirred drinks are never stirred long enough to reach the plateau.  –0.6°C is much more realistic in the real world.
Proof 2: Whether you start stirring right away, or dump ice into a drink and let it sit for a minute before stirring, the drinks will end up about the same. Just throwing ice into a drink doesn’t chill it very much. Because it doesn’t chill very much, it doesn’t dilute very much.
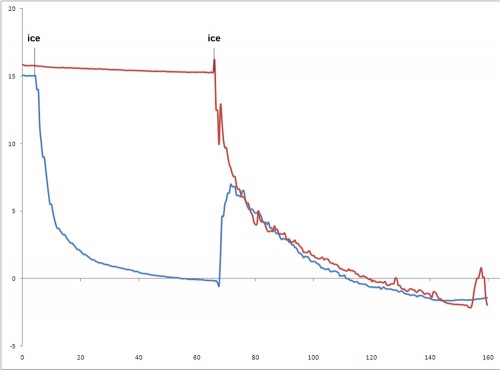
This fact really surprised me. I had assumed that allowing ice to sit in a drink for a minute without stirring would over-dilute the drinks, but both drinks had nearly identical weights when they were finished. You can’t get around physics.
Part 2 coming soon.
still reading, but, re sub-zero ice cooling less quickly: Looks like your curves match but are out of phase. In other words, its slower because it takes a few seconds for the ice to absorb enough energy to go from -20 to 0. During which time, due to the conductivity of the ice, relatively little ice absorbs enough energy to melt. Thus, the ice can’t really start sucking up energy until the whole cube hits zero.
Quite possible.
Wow. Great stuff, and most illuminating. Thanks!
“The Fundamental Law of Cocktails:
Assumption: Bar ice is at 0°C.
Law: There is no chilling without dilution. There is no dilution without chilling. ”
This law is fundamental, true, but only insofar as you’re using water ice to chill a drink. You can get chilling without dilution from other methods, both higher-tech (dry ice, liquid nitrogen) and lower-tech (chilled glasses/shakers, ice-water baths for the shakers/stirrer like a double boiler operating in reverse, or the highly-touted “whiskey stones” which are probably not a good idea to shake unless you enjoy drinking pebbles but probably will stir quite well.)
True, the assumption is you are using standard water ice. LN2 is a zero dilution chiller.
There is an easy way around this:
Don´t let the water reach the cocktail!
If you freeze ice oops, I mean freeze water, in a ZIPlock back and throw that in the cocktail it´ll cool by melting inside, but not dilute.
You will not get below 0°c though cause the freezing point depression effects of the booze don´t hit the ice. Also it will be slower cause the ice doesn’t have this extra incentive of the booze to thaw extra quickly, plus you have a heat transfer barrier in the plastic film.
But it would be doable with a little patience (and maybe an insulated shaker).
Another option would be a double walled shaker: Take 2 conical metal shakers, and maybe little slices of a wine cork to stick those two together in a way the second is half an inch higher than the first.
Fill the gap with water (not quite to the rim) and freeze. Lots of surface, metal as a good heat transfer agent.
When shaking a cocktail in this setup. be sure to wrap a towel around the gap cause you will be rain man otherwise.
Howdy Schinderhannes,
Good to hear from you. In experiments I ran years ago trying to use a Lauda refrigerated cold finger to chill drinks, I found that chilling without the mixing effect of the melt water takes a long time (relative to what is feasible at a bar. It would work fine at home (assuming you had a cold finger)). If you had the time, You could choose the final temperature of your drink by making specific slat water solutions, sealing them in a bag, and freezing those solutions. They should chill below zero to fairly accurate temperatures they will just, as you say, take longer.
” Notice this temperature is a far cry from the -5°C plateau temperature I could have achieved by stirring for a long, long time”
I think you mean -15°C
I’ve never been able to achieve -15C using normal bar ice and room temp 80 proof liquor. Stirring, the best I’ve done is about -7C (finely crushed). Shaken I’ve gotten -8 or so.
Really nice piece, reminds me of my university days in the science labs.
Looking forward to part 2
Eagerly looking forward to part two. Great stuff.
It is obvious to me that I am not stirring drinks long enough. I am glad to know the cube-cracking method was as bad-ass as I suspected.
Questions: Did you measure the final volume or weight of your solution, with, say, your ice size in stirring experiment? I would like to see those differences quantitatively…
You say that stirring a drink does not alter its texture, but what is it about a chilled spirit, perhaps a bottle of aquavit from my freezer, that has such a superior mouth-feel to a room temperature spirit? I know it is a bit of topic, and not addressed in your post, but how far can this be taken? What does a shot of aquavit right above its freezing point feel like in the mouth?
Hello Ian,
I will talk a bit about temperature perception in the part 2. It isn’t as scientific –more just our observations. We have had a lot of ice cold aquavit.
We did measure the weights of our final drinks. The reason I didn’t post them was They weren’t representative of anything because the stirring was carried on much longer than should ever be done. I can try to locate them. I reported weights only on the chart where I stirred both drinks to the same final temperature. I also reported finished abv’s in the old science of shaking II post.
I effing love this blog.
so the question is, what produces the most delicious results. if, we are looking for cold, and no dilution, why not just chill with other mediums? if we need dilution to make something delicious, why not dilute to optimum to begin with and then chill?
these wonderful tests point out our often flawed approach to achieving consistency in taste and temperature. perhaps our whole process is currently wrong in that we can find more efficient and functional ways to achieve delicious results?
btw, what are your thoughts on the frozen rocks which are subbing as ice cubes being sold? are they the answer or just a gimic for stirred or rocked cocktails? could they be an integral part of the process?
A
Howdy Alex,
I haven’t used rocks for chilling –only for heating. They are great for heating drinks but only if you get ones that don’t explode when heated over an open flame (I learned that the hard way). Rocks don’t dilute but they also don’t have that much chilling power. The specific heat capacity of granite (the first rock i found) was only 0.19 cal/gram, whereas water is 1 cal/gram. Even though granite is 2.7 times denser than water, it still doesn’t carry as much power per unit volume per degree –and that is just comparing it to the chilling power of water. It takes 80 cal/gram to melt ice –pretty powerful. They might be effective at maintaining a temperature for a while –I wouldn’t trust them as chillers (but I could be wrong –theory is often disproved by observation).
I agree, this the most intriguing point. So what about something like blast chilled stainless steel cubes or maybe a gel filled stainless steel cube where the gel absorbed the heat conducted by the steel?
Several years ago (like 4 or 5), someone whose name escapes me at CP Kelco developed a freeze-thaw stable gellan ice cube. Presumably the gellan cubes would provide the heat of fusion benefits of normal ice without much dilution. The problem is the liquified water that melts inside the cube isn’t mobile and I think chilling would be inefficient. Not sure though.
Dry ice, anyone?
As mentioned above, there’s a rationale for your negative temp finding by invoking freezing point depression. Interestingly the math works out, too. As most people reading likely know, the idea is that the drink is a solution of ethanol and water, which will have a lower freezing temperature than water alone. It’s the same deal as salt in water where the more solute you have, the lower the freezing temperature of the solvent.
Water drops by -1.86C for each mol solute/kg solvent — here’s a post that runs through an example with isopropanol http://www.newton.dep.anl.gov/askasci/chem00/chem00924.htm
If you replace isopropanol with ethanol instead, you get that a 12% drink freezes at -8C and a 40% drink freezes at -27C. So, the temp you get from shaking may be a function of solute concentration.
On the subject of the perfectly diluted/temp drink — how about bagging a perfectly diluted drink and setting in salted ice water?
Hey Erin,
Re the diluted drink, wait for Part 2.
Freezing point depression isn’t enough to explain why the drink gets colder than zero as you shake it. For instance, many oils have a very low freezing point but if you put an ice cube in them they will only go down to 0 degrees because there is no mixing. The freezing point depression of the fluid and the chilling of the drink is due to a shift in the balance between entropy and enthalpy in the equation governing the melting of the ice.
Absolutely wonderful stuff you’re doing. Can’t wait to see you at Bar Convent Berlin. Na Zdrowie!
Culinology Article on Cocktails –
http://www.culinologyonline.com/articles/mixology-made-easy.html
I would love to see some of these experiments repeated with salty drinks to see how much the electrolyte concentration drops the equilibrium temperature of the drinks.
excellent! thanks for the effort. This answers most of the questions/variables I had after the initial experiments.
Great – really great post. I came across this subject already quite a while – but I am pretty dumb about science and there were a lot of assumptions.
Now I got proof!
Very good work!
This whole series is great. But where were you when I asked this question on Metafilter?
http://ask.metafilter.com/110450/Small-Ice-vs-Big-Ice
I’ve asked the Metafilter mods to post a link to this URL and mark my old question as resolved, but I haven’t seen any activity on that yet.
Cool, thanks.
Wow, I knew that somehow I could combine my engineering degree and love of alcohol! Well done, fellows!